Introduction
Effective evaluations of Antimicrobial Susceptibility Tests (ASTs) require a robust study design. Many methods exist for the in vitro assessment of antimicrobial susceptibility of microorganisms. Susceptibility is determined by evaluating the growth pattern of microorganisms in the presence of an antibiotic. When the microorganism is isolated from a pure culture its inhibition capability is kept in mind. Many new technologies have come up, but technologies to detect antimicrobial resistance is done mostly by detecting the presence of resistance genes or by evaluating the phenotypic response of the microorganism to antibiotic(s) directly in a clinical specimen. The most common methods to detect antimicrobial susceptibility are MIC (Minimal Inhibitory Concentration) determination by Microtitre plate assay and Disc diffusion assay. MIC can be defined as the lowest concentration of antimicrobial agent that is required to inhibit the growth of the organism. Broth and Agar dilution are the most commonly used techniques to determine the MIC of antimicrobial agents against the bacteria under study.
In Agar dilution, the solutions with a defined number of bacterial cells are directly spotted on nutrient gar plates that have different concentrations of antibiotics in them. After the process of incubation, the presence of bacterial colonies on the nutrient agar plates helps us in determining the growth of the organism. If the antibiotic inhibits the growth of bacteria then very fewer colonies will be present and if the antibiotic doesn’t affect the growth of bacteria then more dense colonies will be present.
The difference between agar dilution and broth dilution is that the broth dilution method uses a liquid growth medium containing an increasing concentration of antimicrobial agent which is then inoculated with a defined number of bacterial cells, unlike agar dilution where we make use of solid growth medium. After the process of incubation, the presence of turbidity or sediment indicates the growth of bacteria.
During clinical practice, these methods are used to classify the tested microorganism as clinically susceptible, intermediate or resistant to the tested drug. The Clinical and Laboratory Standards Institute (CLSI) in the USA and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) have published a classification standard to classify the tested microorganism.
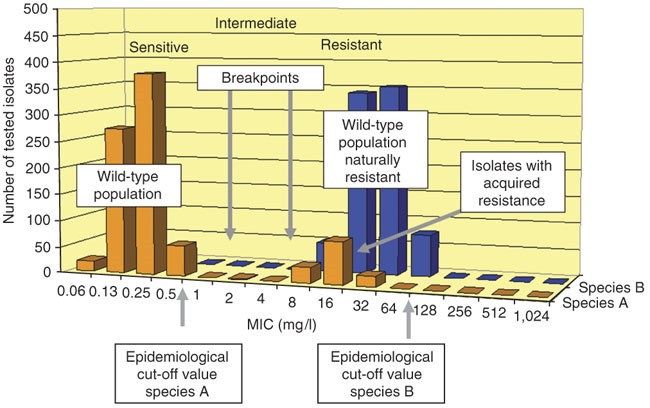
The resistance of the species is mostly associated with therapeutic failure, whereas the susceptibility of the species is associated with a greater probability of a successful association with a therapeutic drug. The highest MIC of the wild-type population is defined as the ‘epidemiological cut-off value’ or wild-type(WT) cut-off value3(Fig. 1). Organisms who have higher resistance can be identified by showing higher MIC value than the epidemiological cut-off value. Determining MIC is a very valuable means to compare as well as for resistance surveillance.
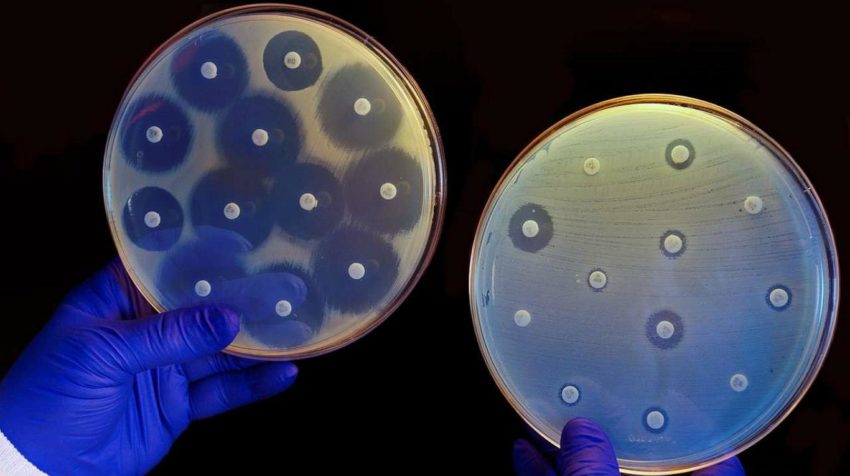
The disk diffusion method given by Kirby-Bauer is a standardized technique for testing rapidly growing pathogens. A standardized inoculum is spread on an agar plate. Mostly, fresh subcultures are used and filter paper disks impregnated with a standardized concentration of antimicrobial agents are placed on the surface and the zone of inhibition is measured around the disk after incubating the agar plates overnight. Specific incubation time ranges are outlined in the Clinical and Laboratory Standards Institute [CLSI] documents.
Principle
In the process to evaluate the minimum inhibitory concentration dilution method a log2 scale is done with each antimicrobial agent in broth that provides a range of concentrations and to inoculate each tube if a microplate is used, then each well containing the antimicrobial agent in broth with a standardized suspension of the microorganism is tested. The lowest concentration given by any antimicrobial agent that inhibits the growth of microorganisms is defined as the minimal inhibitory concentration.
MIC Test
The MIC test can be performed in two ways:-
- Broth Dilution
- Agar Dilution
Materials:
Equipments
- McFarland standard 0.5
- Nephelometer or white paper with black lines
- Microtitre trays with dehydrated antibiotics in two-fold concentrations • Disposable loops (1 µl and 10 µl)
- Multichannel pipette
- Microtitration reader with a mirror
- Disposable reservoir for reagents
- Graduated pipettes (20 µl – 1000 µl)
Broth Dilution
This test concentrates both on Quantitative and Qualitative approach. It can be helpful in knowing the level of resistance of a particular strain and the effect of the decision to use certain antimicrobial agents. The process Broth dilution can be done in 2 ways:-
Macro dilution is a process that uses a broth volume of 1 ml in standard test tubes.
Microdilution is a process that uses about 0.05 to 0.1 ml total broth volume and can be performed in a microtiter plate or tray.
The procedure to be followed for both macro and microdilution are the same except the volume of the broth is varied at times.
Agar Dilution
Preparation of antibiotic stock solution
The stock solution of an antibiotic can be prepared using commercially available antimicrobial powders (potency mentioned) or kits. The amount needed and the diluents in which it has to be dissolved can be calculated by using the following formulas:-

Prepare an antimicrobial agent stock solution at a concentration of 1000 μg/mL or 10 times higher than the concentration to be tested using the above-given formulas.
If you want to get proper results , make sure to sterilize the solution using membrane filters .. Dispense small volumes of the sterile stock solutions into sterile glass, polypropylene, polystyrene, or polyethylene vials; carefully seal; and store (preferably at −60 °C or below, but never at a temperature warmer than −20 °C and never in a self-defrosting freezer). Vials may be thawed as needed and used on the same day.
Preparation of antibiotic dilution range
Make use of sterile 13- x 100-mm test tubes to perform the test. If you need to store the test tubes for later use make sure to freeze them.
Close the test tubes using screw caps, plastic or cotton plugs so that no contaminant can enter the solution present in the test tubes.
Now, take the final two-fold or other dilutions of antimicrobial agent volumetrically in the broth and make the minimum final volume of each dilution needed for the test to 1 mL.
Note: For microdilution of the solution, only 0.1 ml is dispensed into each of the 96 wells of a standard tray to continue the process.
Preparation of Inoculum
Inoculum is prepared by directly making use of broth suspension of isolated colonies selected from an 18 to 24-hour agar plate. The suspension needs to be adjusted to achieve turbidity equivalent to a 0.5 McFarland turbidity standard.
Now, compare the inoculum tube and the 0.5 Mcfarland standard against a card with a white background.
Within the time span, 15 minutes of preparation make sure that you dilute the adjusted inoculum suspension in broth so that after inoculation each tube contains approximately 5 x 10^5 CFU/mL.
Mcfarland Turbidity Standard
This standard given by McFarland is used as a reference to adjust the turbidity of bacterial suspensions in order to see the number of bacteria is within a given range to standardize microbial testing.
| McFarland turbidity standard no. | 0.5 | 1 | 2 | 3 | 4 |
| 1% barium chloride (ml) | 0.05 | 0.1 | 0.2 | 0.3 | 0.4 |
| 1% sulfuric acid (ml) | 9.95 | 9.9 | 9.8 | 9.7 | 9.6 |
| Approx. cell density (1×1^8 CFU/ml) | 1.5 | 3 | 6 | 9 | 12 |
Matching with turbidity standards
The density of the suspension prepared of bacterial cells is compared to the Mc Farland turbidity standard by holding the standard and the suspension in front of light against a white background with contrasting black lines. If the suspension density is too heavy then the suspension needs to be diluted with saline or broth and if the density is not sufficient then suspension needs to be inoculated within 15 minutes.
Preparation of Standard:
Prepare a 1% solution of anhydrous barium chloride (BaCl2).
Prepare a 1% solution of sulfuric acid (H2SO4)
Combine the two solutions and mix the barium chloride and sulfuric acid solutions to form a turbid suspension and BaSO4 in a specific proportion for each McFarland turbidity standard as shown in the above table. Place the mixture in a foil that is covered or using a screw cap for the tube.
When not in use store the standard at a room temperature of 25 degrees Celsius. The density of standard with tie clumps over or precipitates and vigorous vortexing is needed before using it.. Mark the tube to indicate the level of liquid, and check before use to be sure that evaporation has not occurred.

Media
- Sterile normal saline, 4 ml volumes in tubes for nephelometer
- 10 ml cation adjusted Mueller-Hinton II broth in titer tubes
- Nutrient agar plates for purity control of inoculum suspensions Bacterial strains
- Salmonella strains on non-selective agar
Procedure
Day 1
Standardization of inoculum from a pure culture is done. Pick the material from at least 3 to 4 colonies and suspend them in 4ml saline in tubes.
Mix properly and adjust to McFarland 0.5 nephelometer. The instrument should be calibrated before use and gently turn all suspensions upside-down before measuring the contents.
Adjust the turbidity of the inoculum to match that to the given standard and if in case, the nephelometer is not available then compare the standard using white paper with black lines. Compare visually the turbidity of the solution.
Inoculation
Now you need to add 1 ml of the adjusted inoculum to each tube containing 1 mL of the antimicrobial agent in the dilution series and a positive control tube containing only the broth within 15 minutes after the inoculum has been standardized.
This process will result in a 1:2 dilution of each antimicrobial concentration and a 1:2 dilution of the inoculums in the given test tubes.
Agar Plating
In the case of plating on agar plates, the microtitre trays are inoculated with 50 µl of the inoculum suspension using a multichannel pipette or Sensititre autoinoculation. Plates are sealed and incubated at 37-degree celsius for 18-22 hours. Do not stack plates more than 2 degrees high. Purity control is done in which spreading of a 10 µl of the inoculation-suspension on a nutrient agar plate is performed and then those plates are incubated at 35°C overnight.
Incubation:
Make sure to incubate the test tubes at 35 ± 2 ºC for 16 to 20 hours in an ambient air incubator.
Measuring the results
Check the purity of the inoculum suspension. If contamination is present then the results cannot be reported.
To read plates:- Use the record sheet for orientation of the plates, check the growth in the 3 positive control wells. The MIC is measured as the lowest concentration without any visible growth. To check Tubes:- Compare the turbidity of the solution with the given standard.
Interpretation
To measure the antimicrobial susceptibility compare the amount of growth in the wells or tubes containing the antimicrobial agent with the amount of growth in the growth control wells or tubes where no antimicrobial agent is used. A comparison is done among each set of tests when determining the growth endpoints. For a test to be considered valid, acceptable growth (≥ 2 mm button or definite turbidity) must occur in the growth-control well. The lowest concentration at which the isolate is completely inhibited (as evidenced by the absence of visible bacterial growth) is recorded as the minimal inhibitory concentration or MIC.
Disk Diffusion Assay
Procedure for Performing the Disc Diffusion Test
1. Inoculum Preparation
- Growth Method
The growth method is performed as follows
Three to Five well-isolated colonies of the same morphology are selected from an agar plate. To select the colonies, touch the colony with a loop.
Now, transfer the growth into a tube containing 4 to 5 ml of suitable broth medium. The broth culture is incubated until the turbidity is not achieved mostly for 2 to 6 hours at 35 degrees Celsius.
- Direct Colony Suspension Method
In this method, the inoculum can be prepared by making a direct broth or saline suspension of isolated colonies selected from an 18- to 24-hour agar plate (a nonselective medium, such as blood agar, can be used)
2. Inoculation of Test Plates
Mostly, within 15 minutes after adjusting the turbidity of the suspension, a sterile cotton swab is dipped into the adjusted suspension and the swab is rotated many times and pressed against the wall of the tube firmly. This will remove the excess inoculum from the swab.
Now, take an agar plate and streak the cotton swab on it. This procedure is repeated by streaking two more times, rotating the plate approximately 60° each time to ensure an even distribution of inoculum. As a final step, the rim of the agar is swabbed.
The agar plates contain antibiotic discs or filter paper dipped with antimicrobial agents and then after the streaking with cotton swag the plates are left and incubated for colonies to grow.
3. Reading Plates and Interpreting Results
After the time span 16 to 18 hours, the process of incubation is done and then each plate is examined. If the plate was satisfactorily streaked, and the inoculum was correct, the resulting zones of inhibition will be uniformly circular and there will be a confluent lawn of growth. If individual colonies are apparent, the inoculum was too light and the test must be repeated. The diameter of the zones of complete inhibition is measured using the scale. The plates are inverted back and with the help of a ruler, the diameter is measured.
The zone margin should be taken as the area showing no obvious, visible growth that can be detected with the unaided eye.
Faint growth of tiny colonies can be observed or detected only with the help of a magnifying lens that is to be kept at the edge of the zone of inhibited growth, is ignored. Many discrete colonies growing within a clear zone of inhibition should be subcultured, re-identified, and retested for further analysis.

Interpretation:
The MIC and the zone diameter of inhibition are inversely correlated. The more susceptible the microorganism is to the antimicrobial agent, the lower will be the MIC and the larger will be the zone of inhibition. Conversely, the more resistant the microorganism, the higher the MIC and the smaller the zone of The tube dilution test is the standard method for determining levels of resistance to an antibiotic. The lowest concentration of antibiotic which prevents any appearance of turbidity in a solution is considered to be the minimal inhibitory concentration. In the Disc Diffusion test, the zone sizes can differ with reference to the sensitivity pattern. It has been observed that zones of inhibition of a certain diameter can correlate with sensitivity or resistance to the antibiotic tested. In this case, place the metric ruler across the zone of inhibition, at the widest diameter, and then measure from one edge of the zone to the other edge. If there is O-zone present at all, report it as Zone diameter and it is to be reported in millimeters, looked upon the chart, and result reported as S (sensitive), R (resistant), or I (intermediate). The disk diffusion methods are commonly used for routine testing in laboratories and the zone of inhibition guides the right choice of antibiotics that can be used.
References:
- https://www.asm.org/getattachment/2594ce26-bd44-47f6-8287-0657aa9185ad/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Protocol-pdf.pdf
- https://microbeonline.com/preparation-mcfarland-turbidity-standards/
- https://nios.ac.in/media/documents/dmlt/Microbiology/Lesson-12.pdf
- https://microbeonline.com/minimum-inhibitory-concentration-mic-broth-dilution-method-procedure-interpretation/
- https://www.slideshare.net/doctorrao/minimum-inhibitory-concentration