Innovations are flipping conventional antigen-antibody-based assays on their heads. These assays have gained eminence due to their speed, accuracy, sensitivity, the requirement of a minimal sample, and high-throughput conduction. How exciting it is to be a bench scientist these days! Be it for determining a certain antigen/antibody or the presence of coronavirus or HIV in clinical samples; there are immunoassays for detecting and estimating each of these specific antigens or antibodies. But do we know about the working of these assays? This blog will give an insight into the backbone of these assays- Polystyrene Latex Beads and its applications in the medical and diagnostic fields.
What are polystyrene latex beads?
Polystyrene latex beads are spherical polymer particles that are synthesized from amorphous polymers – polystyrene. It is usually synthesized in the colloidal size range. a single particle of polystyrene latex beads contains polymer chains of an average molecular weight of 1X106 gm. These beads are made up of linear hydrocarbon chains that contain benzene rings at every 2nd carbon atom. The rings are responsible for the coiling and entangling of the chain and dominate the space in the particle. The surface of polystyrene latex beads is hydrophobic in nature and provides a surface for strong physical adsorption of molecular species having regions that are hydrophobic in nature, for example, surfactants and proteins. These sub-microscopic polymeric colloidal dispersions have a dual character i.e. they are both colloidal and polymeric. Their stability can be attributed to any one of the two mechanisms:
- Double-layer repulsion – a result of either electrostatic forces (rising from the double layer) or London-van der Waals forces of attraction
- Steric stabilization
Polystyrene latex beads are polymerized in the presence of styrene, an initiator, a buffer, and an emulsifier. The beads can also be made without utilizing an emulsifier. Nevertheless, better results are obtained due to the presence of emulsifier as it has an important role in stabilizing the primary particle and preventing the flocculation by giving a negative charge to the hydrophobic particles. This negative charge may range between 2 to -15uc (microcoulomb) cm-2 and are known to attain the highest charge in an alkaline which in turn increases the stability. Usually, in a latex system, a glycine buffer with a pH of 8.2 is utilized. These particles are known to follow Schultze and Hardy’s law i.e. the nature of the ion that is opposite to the colloidal particle’s charge primarily determines the effectiveness of an electrolyte and the effect increases with an increase in the valency of the ion.
Application of polystyrene latex beads
The most well-known utilization of polystyrene latex beads in today’s era is in the field of immunodiagnostics, especially in latex agglutination tests. This technique is utilized to detect very small amounts of antibody or antigen in cerebrospinal fluids, serum, or urine. It is utilized clinically for identifying as well as typing of several important microorganisms. Latex fixation test of Singer and Plotz (1956) was the first test that was developed with latex which included the coating of polystyrene latex beads by human gammag10bulin and reacting it with antibody to human gammaglobulin that is found in sera of patients with rheumatoid arthritis (RA).
What is a latex agglutination test?
It is an assay that includes mixing of a specific antigen (or antibody) with an antibody (or antigen) in the presence of electrolytes and coating it on the surface of polystyrene latex particles which eventually results in the clumping or agglutination occurring due to the reaction between a particulate antigen and an antibody.
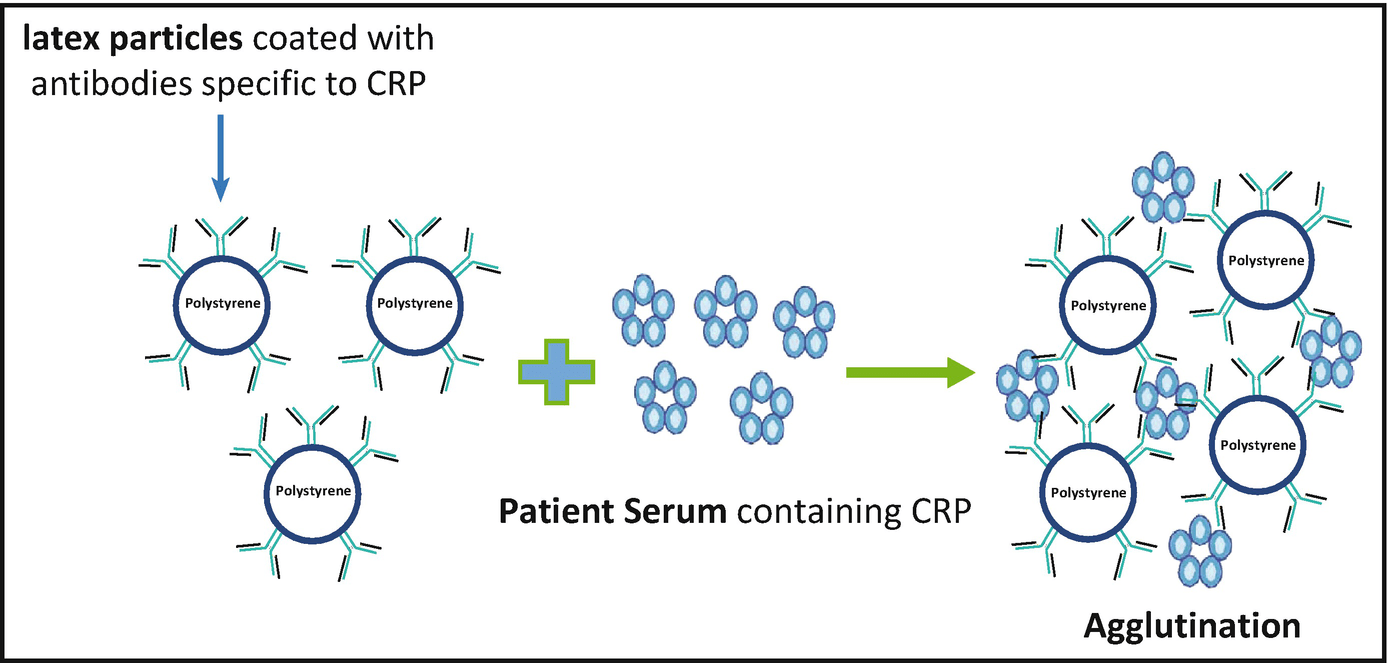
The polystyrene works as a matrix that enables the immobilization of several antigens/antibodies and the binding is hydrophobic in nature which leads to prolonged stability. It serves as a feasible detection of a wide range of substances. Its principle is similar to that of precipitation reactions. The whole process is dependent on the cross-linking of polyvalent antigens. There are three types of agglutination assays:
- Direct Agglutination Test: Antigen agglutinates directly with the antibody
- Indirect or passive agglutination: Antigen is coated on the surface of polystyrene latex beads and the antibody binds to it resulting in the agglutination on the surface of latex particles.
- Reverse passive agglutination test: Includes coating of antibody to the polystyrene latex beads, which helps detect antigen in the patient’s serum.
The reactions are either reported to be positive or negative depending upon the procedure. A positive result is denoted by the evident agglutination of the beads which shows that the patient’s body has produced the pathogen-specific antibody (if the test supplied the antigen) or that the specimen contains the pathogen’s antigen (if the test supplied the antibody). A negative result is denoted by the absence of agglutination which implies that there is no pathogen-specific antibody or antigen in the patient’s body. Few applications of latex agglutination tests include:
-Detection of antigen to Cryptococcus neoformans in cerebrospinal fluid or serum
-Detection of capsular antigens of Haemophilus influenzae, Pneumococcus, and Meningococcus
-Confirmatory test for beta-hemolytic Streptococcus from culture plates
-Detection of Escherichia coli 0157:H7 from suspect colonies of E coli
-Detection of Clostridium difficile toxins A and B
-Detection of rotavirus
-Detection of antistreptolysin O antibody
-Detection of C-reactive protein.
Other applications of polystyrene latex beads in life science and diagnostics include:
– The polystyrene latex beads are utilized as a visual marker in scanning microscope, fluorescent microscope and may serve as a reagent for quantitative as well as qualitative cell-surface and its receptors’ studies.
– In transmission electron microscopy it works as an electron-dense marker and a reagent for cell separation as well. In this process, a magnet separates the beads, on which antigen and/or antibodies are physically adsorbed, from the liquid phase that ultimately results in shortened and simplified carrying out of the procedure.
– The polystyrene latex beads have been employed in phagocytosis studies where these beads are utilized:
- To study the sequential steps of phagocytosis
- To study the kinetic study, rates of ingestion, the effect of metabolic inhibitors, and temperature on phagocytosis
- To study the mechanism of adhesion to membrane and identification of several cells based on surface markers
- To study the effect of a wide range of drugs on the process of phagocytosis
- To study the process of phagocytosis during disease and phagocytosis carried out by platelets
– The polystyrene also latex beads serve as a plain for the adsorption of antigens that are utilized in the form of immunizing agents in animals for enhancing the production of antibodies or in simple words it increases the adjuvancy for antibody production. Some of its miscellaneous applications include:
- Calibration of instruments like Electron microscope modification, shadow angle and thickness of shadowing material, light scattering instruments & techniques, electronic particle counting instruments, optical microscopes, ultracentrifuges, and X-ray diffraction.
- Flagellate locomotion mechanism analysis
- The particle as a model for acoustics, diffusion, electrophoresis, and rheology.
- Aerosolysing particles
To sum it up polystyrene latex beads provide a unique opportunity for simple, reproducible, and rapid immunoassays. Coloured polystyrene latex beads are suitable for “lateral flow assay”, which today is the most used diagnostic procedure. The procedure can be carried out even in areas having limited lab facilitie and is a low-cost alternative method for the estimation and detection of antigens and antibodies.
References:
- https://microbenotes.com/latex-agglutination-test/
- https://www.clinisciences.com/en/read/serological-tests-in-mycology-1190/latex-agglutination-assays-2091.html
- https://www.hindawi.com/journals/jir/2014/850810/
- https://sci-hub.do/https://doi.org/10.1007/978-94-009-3685-0_23
- https://www.thermofisher.com/in/en/home/life-science/cell-analysis/qdots-microspheres-nanospheres/idc-surfactant-free-latex-beads/latex-bead-technical-overview.html#:~:text=A%20spherical%20polymer%20particle%20in,5g%20for%20larger%20particles.
- https://nanoscalereslett.springeropen.com/articles/10.1186/s11671-016-1261-8
- https://pubmed.ncbi.nlm.nih.gov/10945307/
- https://www.researchgate.net/publication/12376096_Application_of_Latex_Beads_Agglutination_Test_for_the_Detection_of_the_Antibody_against_Virus-Infection-AssociatedVIA_Antigen_of_Foot-and-Mouth_DiseaseFMD_Virus