Introduction
Headspace gas chromatography (GC-HS) is a common technique used by analysts for the analysis of volatile organic compounds and their concentrations. This method is reliable, simple and time-efficient that provides sensitivity towards dynamic purge and trap analysis. The ease of operation, diverse uses and economic nature of this technique has made it common among analysts. This technique is generally used in the pharmaceutical industry for the analysis of alcohols in blood and residual solvents in APIs and formulations. It finds applications in the analysis of the monomers of polymers and plastics, flavoured components in beverages and food products and for detecting fragrances in perfumes and other cosmetics.
How headspace gas chromatography helps?
The traditional methods of the analysis result in poor results due to the high molecular weight, non-volatility that may remain suspended in the gas chromatography system. To avoid inaccuracy, many analysts use burdensome sample preparation techniques to extract compounds and avoid unwanted materials. Such techniques are time-consuming and are not economically acceptable. The headspace gas chromatography helps reduce time and cost for sampling. The headspace gas chromatography technique allows analysts to directly sample from the container to extract the volatile headspace.
Principle:
The majority of consumer goods and biological samples are made up of a diverse range of chemicals with varying molecular weights, polarities, and volatility. Headspace sampling is the fastest and cleanest approach for analysing volatile organic chemicals in complicated samples like these. A headspace sample is typically made in a vial that contains the sample, dilution solvent, matrix modifier, and headspace. In the headspace or vapour section of a sample vial, volatile components from complex sample mixes can be separated from non-volatile sample components and isolated.
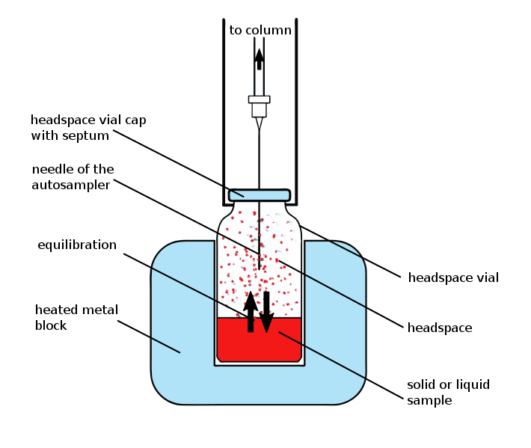
A sample of the headspace vapour is given to a GC apparatus for the separation of all volatile components. When employing headspace/GC, it’s important to pay close attention to sample preparation and instrument setup to get the best results. Minimizing system dead volume, preserving inert sample flow routes, and achieving efficient sample transfer are all important considerations when setting up headspace GC systems.
Water from the sample matrix might also cause difficulties in the transfer line by condensing. Adsorptive areas in the transfer line or injection port caused by the incomplete or inefficient transfer of high molecular weight compounds or water vapour from sample matrices can result in split peaks, tailing peaks, or even irreproducible responses or retention times which can be the cause for faulty results. Using a higher transfer line temperature (125°C–150°C) helps to reduce matrix issues and prevent water condensation from watery samples. To achieve optimum chromatography, high-concentration samples must be properly prepared. Due to sample carryover from prior injections, high-concentration samples can cause ghost peaks in later tests. Thus, it is advised to thoroughly prepare accurate samples with the given norms.
However, higher transfer line and injection port temperatures can reduce sample carryover, although certain samples may need to be diluted and re-analyzed to provide reliable results. To help limit carryover, we recommend injecting standards and samples in order from low to high concentrations. If sample carryover or ghost peaks are seen, bake out the column at its maximum working temperature and raise the temperature of the transfer line to eliminate all of the residual samples. If a sequence of high-concentration samples is expected, running a blank after the suspected samples will reduce carryover contamination of subsequent samples. This helps get good results. It is a preferred lab norm to handle the standard and blanks with the same way the samples are treated, this reduced the chances of inaccuracy and wrong data.
Applications of Headspace gas chromatography
Applications of Headspace gas chromatography includes analysing ethanol and other organic compounds in blood samples and residual solvents in pharmaceutical products and identifying short-chain fatty acids in the given sample.
Solvents for Headspace gas chromatography
In pharmaceuticals, residual solvents are volatile organic compounds used or products while manufacturing drugs and other pharmaceutical products. To detect the impurities and unwanted materials present in the samples, analysts and manufacturers use headspace gas chromatography.
For the workflow of GC-HS, the selection of appropriate solvents is a critical parameter to obtain precise and valid results. The solvents used have an impact on the purity of the sample solvent and on the quality of the chromatogram. The most common GC-HS solvents are water, dimethyl sulphoxide (DMSO), N, N-dimethylformamide (DMF), and N, N-dimethylacetamide (DMAC). N, N-dimethylformamide and dimethyl sulphoxide are specified in Ph. Eur. and USP for water-insoluble substances.
To buy GC-HS grade solvents, please click here.
References:
https://www.thermofisher.com/in/en/home/industrial/pharma-biopharma/pharma-biopharma-learning-center/pharmaceutical-qa-qc-information/residual-solvent-analysis-information.html
https://www.sciencedirect.com/science/article/pii/B0122267702024716
https://en.wikipedia.org/wiki/Headspace_gas_chromatography_for_dissolved_gas_measurement