Originating from China in December 2019, the baneful Corona Virus has wreaked havoc in several countries across the world. As claimed by the World Health Organisation, as on 7th April 2020, globally there are a total of 1,279,722 confirmed cases and 72,614 deaths. In India, on this date, the statistics denote 4643 active cases, 149 deaths and 401 cured as per the Ministry of Health and Family Welfare, GOI. Being under Stage 2 of spreading the infection, with no evidence of community transmission, India has already notified the citizens of the danger. Doctors and researchers are working their tails off to treat those infected, control the disease and find a cure for the same. While most countries are still unprepared for this pandemic, Singapore, Taiwan and Hong Kong’s constant efforts to fight this virus has limited the spread of this disease to a huge degree. They have learnt from the previous corona outbreaks. Pulmonary cells are highly vulnerable to this virus as they express high “lock” protein, which is responsible for the entry and infection of SARS-CoV-2 virus and hence these countries have utilized drugs that act against the viral protein from spreading the further infection inside the pulmonary cells. In defiance of the public health sectors, the governing bodies of almost every country are taking draconian measures to keep the subjects out of danger. India has invested tremendous hard work to fight the Stage 3 community spread of the virus, and develop diagnosis as well as treatment for the disease.
Discussing research in India, the research body ICMR (Indian Council of Medical Research) is working towards the development of effective diagnostic kits and is collaborating with scientists from different institutions and companies for developing vaccines and drugs. Using the available SARS-CoV-2 sequence, also called as 2019-nCoV, RT-qPCR has been used worldwide for detection of the virus. Also, kits are being developed to detect IgG and IgM antibodies in the blood samples against the virus. In India, ICMR is encouraging private and government labs to develop diagnostic kits, which can be sent for approval to NIV and ICMR. The National Institute of Virology (NIV) in Pune also validates all the imported kits from China, Korea, USA before allowing its supply and use for Covid diagnosis.
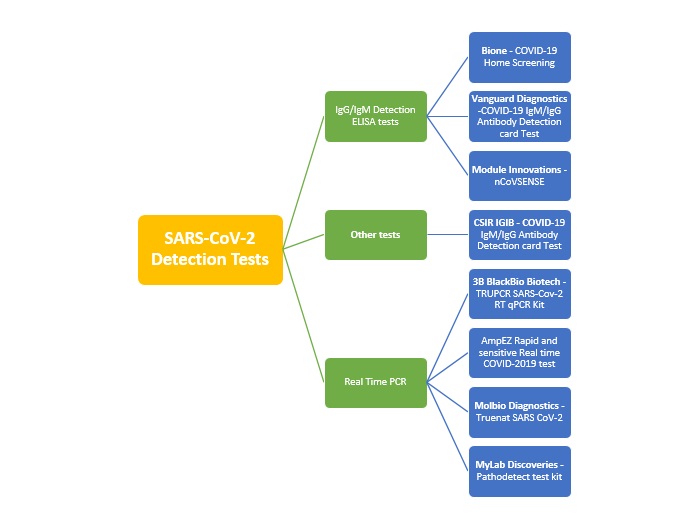
The following is a summary of the different Covid-19 diagnostic kits which have been developed in India, how they work, their price and other details.
- Pathodetect test kit
Type of test – Real Time PCR
Detection time – 2-3 hours
Cost – Rs. 4500
Minal Dakhave Bhosale, R&D Chief, MyLab Discovery has developed the first Indian kit to test COVID-19 in a record 6 weeks. Named “Pathodetect test kit”, this kit takes only 2-3 hours for detection as against 6-7 hours taken by most international RT-PCR kits. The best part, it costs almost 1/4th of international kits, costing at Rs. 4500 per kit. Tremendous hard work and dedication of one virologist have left a crucial benchmark for India to fight against the COVID-19. Pathodetect employs the technique of qualitative PCR for the detection of SARS-CoV2.
- Bione Kits
Under the guidance of their CEO, Dr. Surendra K Chikara, Bione has developed three kits to combat the COVID 19 infection. These three kits use three different strategies to detect or prove susceptibility to the virus.
The COVID-19 Screening Kit
Type of test – Finger-prick test
Test target – IgG and IgM
Detection time – 5 minutes
Cost – Rs. 2,499
An ICMR approved product, this kit allows convenient testing for people belonging to high-risk groups such as diabetic, hypertensive and obese patients and the elderly. The main advantages of the test include the fact that it is a simple finger-prick test, and hence requires no operator assistance. Also, it delivers results within 5 minutes and hence will help speed up the rate of early testing.
The MyMicrobome Kit
Cost – Rs. 14,999
This kit analyses the Gut Microbiome composition, which is an indicator of immunity. This test too is recommended for people belonging to high-risk groups. Bione also offers personalized recommendations regarding diet to improve immunity against the SARS-CoV.
The COVID-19 Genetic Susceptibility Kit
Test target – Detection of genetic variants
Cost – Rs. 7,999
This test is based on the fact that an individual’s susceptibility to a disease is largely genetically determined. The test detects certain genetic variations in an individual’s DNA which could make him more prone to SARS-CoV-2 and the influenza virus.
- TRUPCR® SARS-Cov-2 RT qPCR Kit
Type of test – Real time PCR
The TRUPCR® is Real Time PCR-based test developed by 3B BlackBio Biotech India Ltd. Currently awaiting approval from ICMR, this testing kit allows PCR detection of COVID-19 specific genes. The testing kits also include internal controls which would improve the accuracy of the tests being conducted. Led by Mr. Dhirendra Dubey, the company has released this kit intending to improve the rate of testing by avoiding the costs and time spent in importing kits from other countries.
- TruenatTM SARS CoV-2 (lab-based or near-POC)
Type of test – TaqMan based Real Time PCR
Detection time – 35 minutes
Developed by MolBio Diagnostics Pvt. Ltd., the TruenatTM is a proprietary Real Time PCR platform which promises quicker and more accurate results. Previously designed for the diagnosis of other pathogens like Mycobacterium tuberculosis, MolBio Diagnostics has now adapted this platform to the diagnosis of the novel SARS CoV-2. The test uses a smart microchip platform which requires very low sample volume for providing quick quantitative analysis of the target. The kit also provides sterile tips, tubes and freeze-dried reagents, which allow convenient usage and minimal handling error. Currently, the kit has received a testing licence and is awaiting validation from NIV Pune.
- nCoVSENSe: IgM/ IgG test for spike and N-protein of SARS-CoV 2 (manual)
Test target – IgM/IgG detection
Module Innovations Pvt. Ltd. is a Pune-based start-up focussing on the development of new diagnostic devices. Their earlier products include point-of-care UTI pathogen detecting testing kits – USENSe and ASTSense. The company is now working on a new diagnostic device for the quick detection of the new SARS-CoV 2. The kit detects the IgM and IgG raised against the spike and the N-protein of the virus.
- EyeRa® COVID 19
Type of test – ELISA
Detection time – 5 minutes
This system is being developed by Prantae Solutions, a Bhubaneshwar-based start-up working towards developing novel diagnostic devices. The EyeRa® is the company’s patented technology which is based on ELISA for the detection of pathogens. This platform uses blood serum and yields highly sensitive results within just 5 minutes. The company also supplies a CE compliant SPR device for accurate testing. The product is currently under development and has been granted a test license by CDSCO for evaluation.
- Paper-strip test for detection of the SARS Cov-2
Testing mode – Paper strip test
Detection time – Lower than conventional methods
Cost of the kit – Less than Rs. 500
Developed by a team of scientists at CSIR-IGIB, this paper-strip test is so far the cheapest and most convenient test. Led by Dr. Souvik Maiti and Dr. Debjyoti Chakraborty, the team has developed this test keeping in mind the urgent need to develop easier testing methods compared to the more conventional Real Time PCR. This kit uses the highly sought after CRISPR-Cas9 technology and eliminates the need for expensive equipment and highly skilled labour for conducting tests. It is now seeking approval from ICMR before its release into local clinics.
- COVID-19 IgM/IgG Antibody Detection card Test
Type of test – ELISA
The COVID-19 IgM/IgG Antibody Detection card Test is being developed by Vanguard Diagnostics. The company’s primary focus is haematology and clinical chemistry reagents, Rapid Card Tests, ELISAs and Microbiology based pathogen detection. They have developed their Rapid Card Test system for the quick and easy detection of the SARS-CoV 2. The test aims at detecting the IgM and IgG antibodies raised against the virus using ELISA.
- AmpEZ Rapid and sensitive Real time COVID-2019 test
AmpEZ Rapid and Sensitive Real Time COVID-2019 test is developed by the Ahmedabad-based AmpliGene India Biotech Pvt. Ltd. The company is well-known for novel diagnostic strategies for Point-of-Care Testing. One of their products includes the Genie® II system which uses real-time isothermal amplification for the quantification of both DNA and RNA. They have forayed into COVID-19 testing through the AmpEZ system which sensitive results in a low time frame.
The following diagram is a summary of the available tests for the diagnosis of SARS CoV-2:
While India gears up for Covid-19 diagnosis, a few top companies are racing towards the development of vaccines against Covid-19. Gujarat based Zydus Cadila and Pune based Serum Institute have collaborated with America’s Codagenix on the project of making vaccines, which are currently in the pre-clinical stage. Zydus Cadila is testing a DNA vaccine that will target viral membrane protein that allows for the entry of virus into human cells. Serum Institute is working on a ‘tailor-made’ live virus incapable of causing infection, i.e. live attenuated vaccine. With the help of computational tools, researchers from the University of Hyderabad are predicting that potential vaccine could be prepared by employing T-cell epitope which will act against all the structural and non-structural protein of virus. Dr. Seema Mishra, faculty at the School of Biochemistry, University of Hyderabad has designed such vaccine candidates, essentially small coronaviral peptides, with the potential to trigger an immune response against the peptides. These vaccine candidates have been disseminated to the biological labs for urgent experimental analysis.
While the transfer of vaccines from labs to clinics will take about 2 years, it shall be helpful in the long run. Currently the best way is prevention by social distancing, cleaning of hands and surfaces, wearing masks and staying at home. Hence, the Government of India is spending its major resource and attention to stopping the spread of this viral contagion and break the cycle of propagation.
References:
- https://economictimes.indiatimes.com/industry/healthcare/biotech/healthcare/meet-the-woman-behind-indias-first-Covid-testing-kit/articleshow/74857787.cms
- https://m.hindustantimes.com/india-news/coronavirus-update-india-inc-joins-race-to-make-drugs-and-vaccine/story-EZNu1BpAfb337tZIuDDQxM_amp.html
- https://www.nature.com/articles/d41587-020-00010-2?_lrsc=71224024-9e34-44e8-92ac-d9a2e72505be&utm_source=linkedin+elevate&utm_medium=social+media
- https://the-ken.com/story/the-two-indian-companies-expanding-the-scope-of-Covid-19-testing/?searchTerm=Covid#
- https://www.thehindubusinessline.com/companies/two-indian-companies-in-race-for-vaccine-against-Covid-19/article31025613.ece
- https://economictimes.indiatimes.com/news/politics-and-nation/centre-backs-vaccine-trials-to-find-a-cure-for-Covid-19/articleshow/74856770.cms
- https://economictimes.indiatimes.com/news/politics-and-nation/niti-aayog-stakeholders-discuss-rd-requirement-for-coronavirus-vaccine/articleshow/74695765.cms
- https://www.thehindubusinessline.com/news/science/uoh-faculty-designs-potential-Covid-19-vaccine-candidates/article31182935.ece#
- http://www.ijmr.org.in/preprintarticle.asp?id=281471;type=0
- https://www.bione.in/
- https://www.3bblackbio.com/index.html
- http://www.ampligeneindia.com/about.php
- http://www.molbiodiagnostics.com/
- http://moduleinnovations.com/
- https://www.mumbailive.com/images/media/images/images_1585069714923_ET4yosZU4AE1XYo.jpg?bg=ccddeb&canvas=0.25&crop=680%2C381.7543859649123%2C0%2Cnull&fit=crop&fitToScale=w%2C1368%2C768&h=303.1578947368421&height=679&w=540&width=680