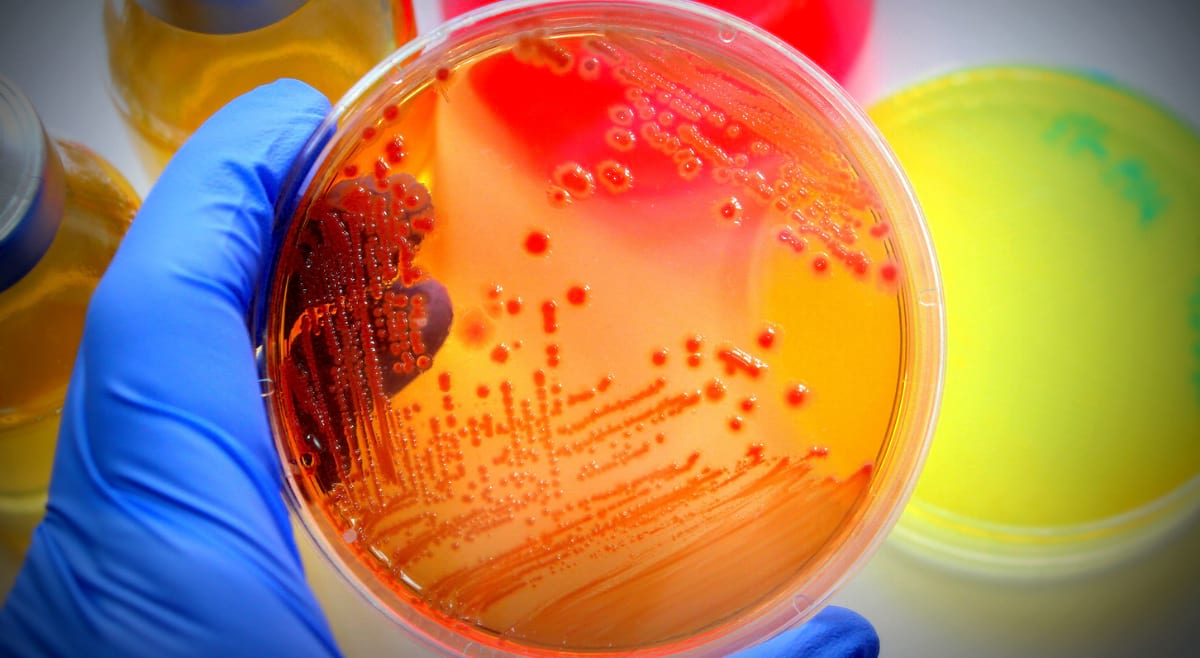
Handling bacterial cultures can be troublesome sometimes. We have brought a lucid guide to using commercially available bacterial cultures. KWIK-STIK™, KWIK-STIK™ Plus and LYFO DISK™.
KWIK-STIK™, KWIK-STIK™ Plus and LYFO DISK™ microorganisms are used to test the performance of assays, reagents, and other media that are used in microbial testing for identification and detection of cultured microorganism isolates. These are particularly designed for QC in laboratories, for presence-absence testing, microbial identification methods, antimicrobial susceptibility testing, etc.
How to use KWIK-STIK and KWIK-STIK Plus Microorganism
KWIK-STIK and KWIK-STIK PLUS are used for their clever design and reliability. They contain single QC microorganism strains in lyophilized pellets. They come in convenient packaging which is ready to use.
1. Equilibrate unopened KWIK-STIK pouch at room temperature. Tear open the pouch at the notch and remove the KWIK-STIK unit.
2. Tear off the Pull-Tab portion on the label and attach it to the primary culture plate. Remember not to disassemble the device during hydration.
3. Carefully, crack the ampoule of the KWIK-STIK to release the hydrating fluid.
4. Tap vertically on a hard surface of the counter to facilitate the flow of the fluid.
5. Pinch at the bottom of the unit to crush the pellet in the fluid making it homogenous.
6. Thoroughly saturate the swab with the hydrated material and transfer to the appropriate agar medium or as per your lab’s SOP.
7. Inoculate the culture plate(s) by gently rolling the swab over one-third of the plate.
8. Facilitate colony isolation by using a sterile loop.
9. Discard the KWIK-STIK using proper biohazard disposal.
10. Incubate the inverted inoculated primary culture plate(s) at favorable conditions.
LYFO DISK Microorganism Procedure
These are lyophilized pellets containing a single strain of microorganisms. These are much more economical references for stock culture. You may simply rehydrate the pellets and inoculate. These are available as re-sealable vials consisting of 6 pellets of QC microorganism strain.
How to handle them?
1. Equilibrate unopened LYFO DISK vial at room temperature.
2. Remove 1 pellet with sterile forceps from the vial. Do not remove the desiccant.
3. Place the pellet in 0.5 ml of sterile fluid. Quickly recap the vial and return again to put in 2°C to 8°C storage.
4. Crush the pellet with a sterile swab to make it homogenous. Saturate the same swab with the hydrated material and transfer it to an agar medium.
5. Inoculate the primary culture plate(s) by gently rolling the swab over one-third of the plate.
6. Facilitate colony isolation by using a sterile loop.
7. Discard the LYFO DISK using proper biohazard disposals.
8. Immediately incubate the inverted inoculated primary culture plate under appropriate conditions.
To recover phage from freeze-dried or thawed liquid nitrogen vials:
- Prepare an actively growing early log phase broth culture of the recommended host strain before opening the phage specimen.
- Add 0.25 ml of the host broth to a freeze-dried phage. Then pre-warm the plates in an incubator.
- Layer 2.5 mL melted 0.5% agar (same medium) that contains one or two drops of the freshly grown host maintained at 43°C to 45°C until ready to pour. Allow it to dry and harden.
- Serially diluted the re-hydrated phage using a 1:10 dilution scheme by passing 0.25 mL of the phage into a tube containing 2.25 mL of the broth medium for freeze-dried vials.
- Similarly, add 0.5 mL of the phage into a tube containing 4.5 mL of the broth media for frozen vials. Repeat the passages as per the requirements.
- Spotted one drop of each dilution on the surface of the prepared plates. Let it dry. On each of the plates, three to four dilutions can be placed. Lysis might be visible after 24 hours of incubation.
We hope these instructions help you carry out experiments with ease. If you have any questions then make sure to put them in the comments section. We are happy to help!